In 2022, Basser Center co-founders Mindy and Jon Gray announced a $55 million gift to launch the new Basser Cancer Interception Institute (BCII). This Institute aims to dramatically disrupt the timeline of cancer treatment, intercepting cells as they begin to develop into very early cancers, and halt or reverse that process. Basser Executive Director Susan Domchek, MD and her colleagues are launching many initiatives for the new Institute, such as leading a study testing a cancer vaccine in men and women with BRCA1 and BRCA2 mutations.
Interception in Action at the Basser Center
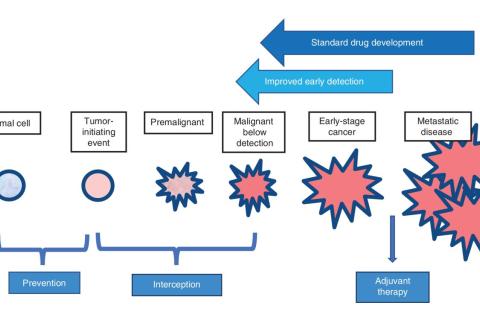
What is cancer interception?
Cancer interception involves intervening in some way at the earliest stages of cancer formation, when the very first abnormal cells develop. You can think of it as periodically weeding a garden. By removing these early cells before they have a chance to grow, it may be possible to stop cancer from ever developing.
The ultimate goal of the BCII is to discover as many potential methods and interventions to intercept these cells as possible.
Cancer Prevention Vaccine Clinical Trial
Dr. Domchek spearheaded the BCII-funded study aimed to intercept cancer by pushing the immune system toward recognizing and destroying early abnormal cells before they grow into full-fledged cancer. This strategy involved using DNA medicine, developed with Philadelphia biotech company Inovio Pharmaceuticals, to train the immune system, specifically T cells, to recognize an enzyme called telomerase that’s involved in about 95 percent of human cancers.
BCII-Funded Research Projects
The Basser Cancer Interception Institute funds many research studies related to cancer interception across Penn Medicine and external organizations. The following selection of research initiatives illustrates the breadth and promise of our work—with many other projects advancing behind the scenes.